INTRODUCTION
Dementia is a global public health problem affecting approximately fifty-five million people. Due to the aging population, this figure is expected to rise to 152 million by 2050 (Nichols et al., 2022). Alzheimer’s disease (AD), a neurodegenerative disorder characterized by progressive memory deficits and disability, is the leading dementia sub-type (DeTure & Dickson, 2019), for which there is currently no cure. In addition to vascular prevention, the current pharmacological approach involves FDA-approved symptomatic therapy that provides limited long-term benefits (Alzheimer’s Association, 2016). Approximately 80% of those with cognitive impairment will experience the behavioral and psychological symptoms of dementia at some stage of the disease (Lyketsos et al., 2002). Both dementia patients and their caregivers can benefit from adequate symptom management.
Several non-pharmacological interventions have been proposed to manage neuropsychiatric symptoms and even improve cognitive function to improve the quality of life of patients and caregivers (Livingston et al., 2014). Three types of non-pharmacological cognitive interventions have been developed to improve cognitive functioning in people with dementia. Cognitive stimulation involves non-specific exercises, tasks, and activities such as word games and brain teasers focused on cognitive and social functioning reinforcement (Berg-Weger & Stewart, 2017). Reminiscence therapy in people with dementia, using photographs and everyday items such as music and movies, encourages participants to talk about past experiences and memories, promoting skill retention and mood improvement (Huang et al., 2015). Cognitive training seeks to maintain or improve a particular aspect of cognitive functioning (such as memory or attention) through structured, guided practice provided individually or in a group (Bahar-Fuchs et al., 2013). Cognitive rehabilitation is an individualized intervention focused on a person’s needs and includes models that help slow down cognitive decline. Minimizing mental decline also involves using strategies to find, learn, and practice compensation techniques (Choi & Twamley, 2013). Individuals with cognitive impairment and therapists usually work together to achieve key goals (Wilson, 2002). However, a systematic review has confirmed that a single intervention implemented in a group setting does not suffice to meet all a patient’s needs. Each person with dementia must be individually assessed and treated accordingly (Meyer & O’Keefe, 2018). Ideally, clinical interventions, often achieved through collaboration between the patient and a close family member, must be tailored to the individual’s priorities, needs, and preferences. Individualized intervention is characterized by being delivered by the same trained therapist who knows the diagnosis, stage, and individual characteristics of the person receiving the intervention.
Non-pharmacological interventional studies are characterized by distinct levels of evidence and heterogeneous methodologies. The authors of a literature review stated that these programs were at best comparable to traditional care (Petriwskyj et al., 2016). To our knowledge, there is only one randomized controlled trial (RCT) of individualized cognitive rehabilitation, involving both a person-centered approach and high-quality evidence targets. The Efficacy Assessment of Three Non-Pharmacological Therapies in Alzheimer’s Disease (ETNA3) Trial compared the impact of cognitive training, reminiscence therapy in group sessions, and an individualized cognitive rehabilitation program with individualized sessions on the rate of progression of dementia. A two-year follow-up analysis of the 653 AD outpatients who received the latter intervention reported no impact on primary efficacy measures (moderately severe to severe dementia-free survival rates). Moreover, none of the secondary outcomes (such as impairment, functional disability, behavioral disturbance, quality of life, depression, caregiver burden, and resource use) involving group session interventions differed from those of usual care. However, with an individualized cognitive rehabilitation program, reduced functional disability, and a six-month institutionalization delay at two-year follow-up were observed (Amieva et al., 2016).
Given this evidence, it is essential to determine whether these practices are applicable in settings outside clinical research and in other sociocultural contexts. Countries in Latin America have different resources, cultural and historical features, ethnicities, socioeconomic disparities, and health and economic systems (Aravena et al., 2022). The main objective of this study was therefore to evaluate the feasibility, acceptability, retention, adherence, and satisfaction rates of an individualized cognitive rehabilitation program based on the French ETNA3 study, administered to mild dementia outpatients at a memory clinic in Mexico City.
METHOD
Participants and setting
We conducted a single-arm clinical trial at the memory clinic of a tertiary-level hospital in Mexico City with non-institutionalized subjects over fifty-five years of age with a diagnosis of mild Alzheimer’s disease (AD). The same evaluator (a certified geriatrician or neurologist) performed a clinical and cognitive evaluation using the NINCDS ADRDA (McKhann et al., 2011) and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria to determine possible or probable AD (Sachdev et al., 2014), whether the person had a caregiver (someone who has contact with the subjects for at least ten hours a week), and the absence of uncontrolled and/or concomitant depression or vascular dementia.
Exclusion criteria included subjects who were unable to read or write, had major depression on the Geriatric Depression Scale, (GDS) )( or equal to 6 points, a Hachinski 7 single-item scale score )( 2 ( 2 or more indicates a vascular component of cognitive impairment) (Hachinski et al., 2012), with poor chronic disease control (such as heart disease, hypertension, lung disease, liver disease, cancer, and diabetes) or altered vitamin B12 or vitamin D levels.
Since this was an exploratory feasibility study, no sample size calculation was performed, with researchers simply recruiting patients who met the inclusion criteria and agreed to participate in the period from December 2018 to February 2019.
Recruitment
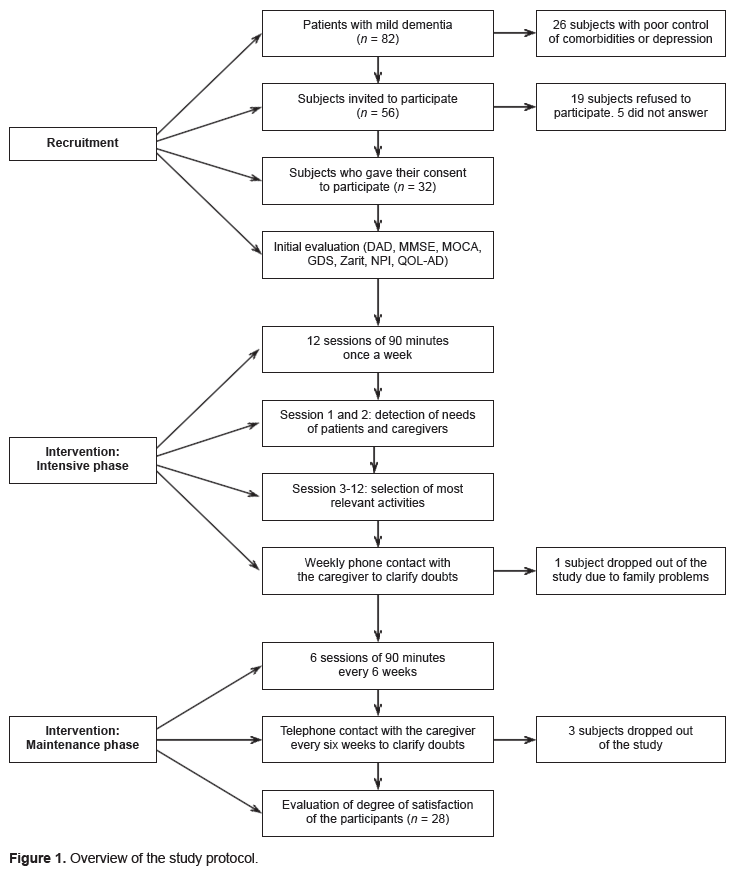
To determine eligibility, researchers reviewed subjects’ records at the memory clinic. Subjects who met the inclusion and exclusion criteria were subsequently contacted via telephone to receive an explanation of the study’s characteristics and an invitation to participate. If a person was interested, we scheduled an appointment to confirm their eligibility and ask further questions. Both the subject and caregiver gave their informed consent in writing. The local Ethics Committee approved this protocol.
Figure 1 provides a summary of the protocol followed for recruitment. Eighty-two subjects with mild dementia were initially identified. After a review performed by researchers at the memory clinic for eligibility criteria, only fifty-six were invited to participate. Finally, thirty-two subjects and their caregivers gave their written consent.
Intervention
The intervention process followed a standardized procedure to guarantee homogeneity. Three psychologists with experience in the field of dementia were recruited. They received a three-day training program taught by ETNA3 study staff (Amieva et al., 2016). A detailed explanation of the rehabilitation plan and a manual specifying the intervention guidelines were provided. Monthly supervision sessions were also conducted during the study.
The cognitive rehabilitation program consisted of ninety-minute sessions. As shown in Figure 1, during the first three months, these sessions were scheduled weekly (intensive phase), while maintenance sessions were held every six weeks for the following nine months. All evaluations were performed in a doctor’s office by the same psychologist originally assigned to each patient.
The cognitive rehabilitation therapy consisted of a program conducted through individual sessions (with the subject and caregiver). The psychologist(s) asked the patient to choose the therapy for each day, after considering their clinical characteristics, including sociodemographic factors, severity of the disease and cognitive and behavioral alterations. In the first two sessions, the following were explored with the patient and their caregiver: the activities (hobbies or crafts) the patient used to enjoy and had stopped doing; the patient’s interest in recovering and talking about their life story, from their oldest memories (childhood) to their current ones (reminiscence therapy). It was agreed that the material required to perform each activity would be provided by the patient and their caregiver (such as photographs, sewing materials, and books). The first two sessions were dedicated to selecting tasks related to leisure or daily activities according to the personal objectives and cognitive abilities of each subject. In both therapies, we emphasized the engaging in everyday activities to find strengths that would compensate for deterioration, such as tasks that involve learning information. (such as lists of words, stories, and figures) and the discussion of past activities, events, and experiences enhanced by items such as photographs, familiar items, songs, and personal recordings (Amieva et al., NCT00646269). In the following sessions involving individualized therapy, the patient decided what to do in each session: cognitive therapy or reminiscence.
Case examples
Case 1: A seventy-nine-year-old housewife, who compulsively washed clothes even though they were clean, all day every day. She had previously enjoyed embroidery, “but I don´t know how to do it anymore.” She chose cognitive therapy and brought in her unfinished embroidery material. We began to work by trial and error, emphasizing successes and downplaying mistakes. At the same time, we remembered the names of stitches, threads, and lace. The woman stopped washing clothes and began to spend her time embroidering. Case 2. An eighty-five-year-old man, previously engaged in public activity with a large support network for agenda management. Although his main interest had been social activities, he now lived in a state of anhedonia and apathy. Since he had no interest in cognitive activities, he chose reminiscence therapy. We began by talking about his career using positive material obtained from the web. The caregiver provided photographs from his childhood, youth, and adulthood, facilitating the intervention. We also used music and artwork from the Web. His family started to become involved and he began to participate more.
During the first three months, the psychologists contacted the caregivers weekly, urging them to ask questions about the intervention and encouraging them to mention any particular difficulties. They were subsequently called every six weeks during the maintenance phase of the program.
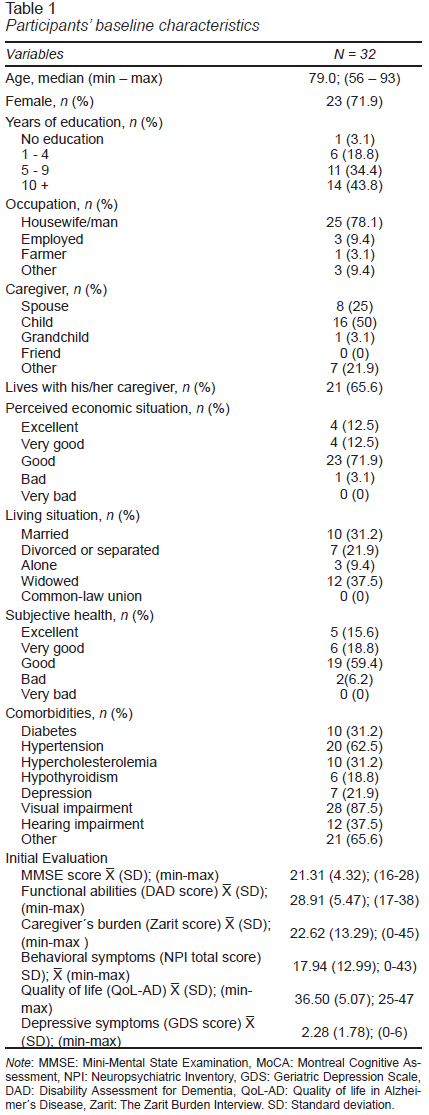
Subjects’ demographics (age, sex, education level, occupation, perceived financial status, subjective health status, relationship with caregiver, and living situation) and comorbidities were recorded at baseline. Their cognitive status was evaluated using the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) and the Montreal Cognitive Assessment (MoCA-S) (Aguilar-Navarro et al., 2018). Behavioral symptoms were evaluated using the Neuropsychiatric Inventory (NPI) (Cummings et al., 1994), depressive symptoms using the Geriatric Depression Scale (GDS), (Yesavage et al., 1982), functional capacity with the Disability Assessment for Dementia (DAD), (Gélinas et al., 1999), and quality of life through Quality of Life in Alzheimer´s Disease (QoL-AD) (Logsdon & Teri, 2018), while the caregiver burden was assessed with The Zarit Burden Interview (Zarit et al., 1986).
Feasibility and Acceptability Evaluation
Feasibility was determined by evaluating the eligibility, acceptability, retention, adherence, and tracking rates of the method. We also evaluated the number of adverse effects and degree of satisfaction. These parameters are described below:
Eligibility rate: Calculated by dividing the number of people diagnosed with mild Alzheimer’s dementia at the memory clinic by the number of subjects meeting the inclusion criteria.
Acceptability rate: Calculated by dividing the number of people who gave their written consent to participate in the study by the number of subjects diagnosed with AD who met the inclusion criteria and were invited to participate.
Retention rate: Defined as the number of people who remained in the study, in other words, the number of participants who did not drop out of the study divided by the total number of participants recruited.
Adherence rate: Measured by adding the total number of intervention sessions received by participants divided by the total number of sessions scheduled for the participants included (eighteen sessions for each subject).
Adverse events: Researchers recorded any adverse events perceived by participants during the intervention, such as emotional distress, acts of aggression during sessions, or changes in emotional functioning.
Satisfaction: Assessed in subjects and their caregivers through a six-item questionnaire on their participation in the study, the information provided, the availability of the researchers, activities conducted in the sessions, frequency of sessions, and logistical organization rated on a Likert-type scale ranging from one (not at all satisfied) to seven (Totally satisfied). The minimum score was six points, and the maximum score forty-two.
The study was feasible if the rates had a value of over 50%, as in those reported in previous studies (Orsmond & Cohn, 2015).
Program effectiveness
Evaluations were conducted at two moments: at baseline and twelve months (at the end of the maintenance phase).
Cognitive status was assessed using the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MOCA) (Folstein et al., 1975; Freitas et al, 2013). Behavioral symptoms were evaluated using the Neuropsychiatric Inventory (NPI) (Cummings et al., 1994), depressive symptoms using the Geriatric Depression Scale (GDS) (Yesavage et al., 1982), functional capacity using the Disability Assessment for Dementia (DAD) (Gélinas et al., 1999), quality of life using the Quality of Life in Alzheimer’s Disease (QoL-AD) (Logsdon & Teri, 2018), and caregiver burden using The Zarit Burden Interview (Zarit et al., 1986) at these two points in time.
Data analysis
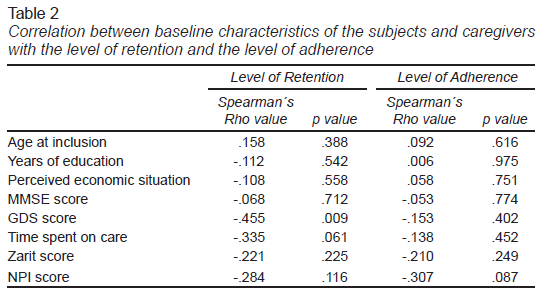
Categorical variables were described as numbers and proportions, and continuous variables as means, standard deviations (SD), and ranges. We determined the rates of eligibility, acceptability, retention, and adherence, as well as the adverse effects and the subject’s degree of satisfaction. We performed a correlation analysis to establish an association between the demographic and clinical variables and the feasibility values (number of sessions and assessments completed) with Spearman’s rho test.
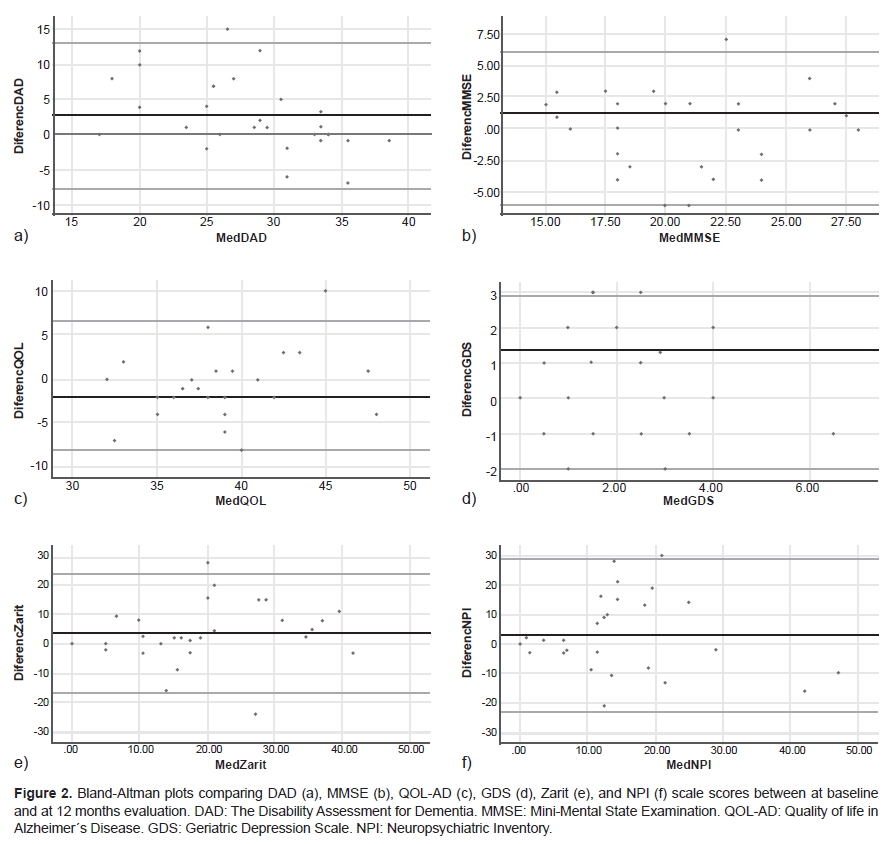
Using the Shapiro-Wilk test, we found that the variables did not have a normal distribution. For comparisons before and after the cognitive rehabilitation program (at baseline and twelve months), we used raw scores for all analyses and the Wilcoxon nonparametric test (Dexter, 2013). The effect of the intervention was also based on case studies. Individual scores were plotted for each participant’s comparison between baseline and twelve months to visually compare the distribution of results among participants who completed the program, allowing for person-based assessment. Global scores were calculated for each questionnaire and subscale (DAD, MoCA, MMSE). Field notes were collected at the end of each visit and after telephone conversations to record participants’ experience with the protocol and any feedback. The efficacy of the intervention and feasibility of the protocol and intervention were evaluated based on the case studies (Blampied, 1999; Thomas, 2011).
The statistical analyses were performed using SPSS vs. 25.
Ethical considerations
This study protocol was reviewed and approved by the local Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, (INCMNSZ), approval number [NER-2465]. For this study, both the subjects (or their parent/legal guardian/next of kin) and their caregivers gave their informed consent in writing.
RESULTS
A total of thirty-two persons living with a major neurocognitive disorder and their caregivers agreed to participate. Table 1 shows the baseline characteristics of the participants. The mean age was 80.03 ± 7.8 years, 72% were women, 78% had completed five or more years of education, and 22% were still working. Twelve subjects were widowed (37.5%), and ten married (31.2%). Most of the caregivers were either offspring (50%) or spouses (25%). Twenty-one participants lived with their caregivers (65.6%). Most of the participants reported good to excellent health (93.7%). The most frequent comorbidity was corrected visual deficit (87.5%), followed by hypertension (62.5%) (Table 1).
At baseline, the mean score for the scales of MMSE was 21.3. ± 4.3 points, MoCA 14.38 ± 5.3 points, DAD 28.9 ± 5.4 points, Zarit 22.6 ± 13.2 points, NPI 17.94 ± 12,9 points, Qol-AD 36.5 ± 5.07 points, and GDS scale 2.28 ±1.7 points respectively. Of the 28 participants who completed the study, 15 (53%) of the total number of participants showed overall cognitive improvement, (MMSE and MoCA), with seven (25%) participants improving their independence in basic and instrumental activities on the daily activities scales (DAD). However, this did not achieve statistically significant levels (Figure 2).
Of the eighty-two cases with AD discussed at the memory clinic, fifty-six met the inclusion criteria and were contacted. The calculated eligibility rate was 68%.
The acceptability rate was 57%. Of the fifty-six subjects contacted, thirty-two agreed to participate (57%), nineteen declined (34%), and five did not respond (9%).
The retention rate was 87.5%, with 32 subjects entering the study. Four subjects withdrew from the study for various reasons (health issues, the caregiver could not take the subject to the sessions, family problems, and loss of follow-up). In the correlation analysis, a lower initial score on the GDS was associated with a higher retention level. The adherence rate of the twenty-eight subjects who remained in the study was 100%. We planned eighteen sessions for each subject included, providing five hundred and four sessions, some of which were rescheduled due to logistical difficulties or health issues. The caregivers remained highly engaged throughout the program and responded to scheduled follow-up calls. A low negative correlation was observed between baseline behavioral symptom scores and level of adherence, although this was not statistically significant (Table 2).
Subjects or caregivers reported no adverse effects during the intervention.
The mean score on the satisfaction questionnaire for the 28 subjects with dementia who completed the study was 39.7 ± 8 points (representing 94.7% satisfaction) and 40.7 ± 2.2 points for the caregivers (representing 97.1% satisfaction).
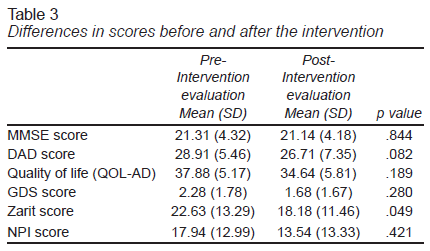
Table 3 presents the mean for each outcome measure before and after treatment. Wilcoxon tests revealed no significant differences in measures of cognition (p = .414), quality of life (p = .189), depression symptoms (p = .28), or neuropsychiatric symptoms (p = .421). A lower caregiver burden score was observed, however, which was significant (p = .049).
DISCUSSION
Feasibility studies can help researchers predict whether a future trial is likely to be productive. This study determined the feasibility of an individualized cognitive rehabilitation program derived from the French ETNA3 study at baseline and 12 months. A previous review of studies conducted in Latin America has reported similar barriers to this study: the cultural assumption that the families of people with dementia should care for them, meaning that their needs are overlooked (Aravena, 2022). Resources vary widely, since in Mexico, there are limited funds and human resources to be able to replicate studies. There is a low rate of diagnosis of dementia in its early stages compared to developed countries as it is considered a normal aging process due to the low level of information on AD, even among the relatives and caregivers of patients with dementia, with treatment focusing on pharmacological measures. In Latin America, the diagnosis is usually made by specialists and only occasionally by a general practitioner (GP), in contrast to European countries, where most patients are diagnosed by a GP (Nitrini, 2020).
In this study, the eligibility rate was 68%, and causes of exclusion were poor control of chronic diseases, concomitant vascular dementia, and depression, reflecting the high degree of comorbidity in the Mexican population, which could play a confounding role in the lack of response to non-pharmacological treatment of dementia.
The study acceptance rate was 57.1%. Understanding the reasons for non-participation in interventional studies is essential because information obtained through these assessments can improve consent rates in future studies. Similar recruitment rates of 49.2% have been reported in previous behavioral trials involving patients and a support person (Trivedi et al., 2013). The most common reasons for not giving consent for physical activity programs are pre-existing medical conditions and lack of interest (Hubbard et al., 2016). Recruiting dyads poses a challenge because both subjects (patient and caregiver) must meet the eligibility criteria and be willing to participate in the sessions planned throughout the trial. A major challenge is encouraging eligible subjects to participate in the study and maintaining engagement in the activities due to the presence and severity of pre-existing diseases, the lack of awareness concerning non-pharmacological interventions, and the existence of misconceptions regarding the value of treatment programs with low expectations of success (Clare et al., 2004; Wigfield & Eccles, 2000).
Participants’ level of adherence is an essential component of successful interventional programs, which not only involve training subjects and educational sessions but also the involvement of caregivers and family support systems. Moreover, for cognitive rehabilitation to be attractive and practical, participants’ feelings of self-competence and self-efficacy must be enhanced. These patients could benefit from cognitive behavioral therapy targeting feelings of hopelessness and low expectations of success. In this study, the adherence rate was 100%, which is higher than the average reported in previous studies (Trivedi et al., 2013). This suggests that subjects remained highly involved throughout the study, due to the close support of their caregivers, who provided a basic level of stimulation. In our research, in addition to educating caregivers about neurocognitive disorders, it was also essential to encourage them to answer questions or share experiences with their patients or each other. This was done at the end of each of the sessions individually. Subjects who dropped out of the study had more behavioral symptoms, which could have contributed to differences between participants and caregivers and prevented them from continuing with the study. It has previously been reported that, together with the self-perception of worsening health and longer care time, dropout rates are associated with the physical and psychological problems of caregivers, which could prevent them from taking the person with dementia to the center for rehabilitation sessions (Custodio et al., 2014). More depression symptoms were observed among subjects who failed to complete the planned sessions, as has been reported in studies of predictors of treatment adherence (Gebrie & Ford., 2019).
In this study, although no statistically significant differences were achieved in the overall cognitive evaluation due to the statistical power of the sample, some individual changes of improvement are observed in the case-by-case study, with stability in the mean scores at twelve months. The neural mechanisms underlying the positive influence of non-pharmacological treatment remain unclear. Enhanced brain plasticity, the brain’s ability to restructure itself in response to stressors, may be an essential component of the mechanism responsible for the cognitive improvements associated with rehabilitation interventions. In one study, serum brain-derived neurotrophic factor (BDNF) levels increased in the intervention group compared to basal conditions, which was also related to cognitive improvements (Jeong et al., 2016). The latter results suggest that functional deficits could be offset by improving the efficiency of the neural network (Shigihara et al., 2020).
In this study, a significant decrease in caregiver burden was observed, suggesting that caregivers feel less tired helping their patients, together with a decrease in behavioral symptoms that was not statistically significant (Kurth et al., 2021).
Despite potential side effects, drug treatments dominate the dementia treatment landscape. In particular, antipsychotics are associated with an increased risk of stroke and death (Ralph & Espinet, 2018). The efficacy of non-pharmacological therapy in improving cognition or slowing down the progression of cognitive decline has been confirmed, which is why non-pharmacological therapy constitutes a potentially effective complementary treatment for dementia (Wang et al., 2020).
Since the evolution of neurocognitive disorders is heterogeneous, treatment approaches must be individually tailored. Interventions should ideally be designed for both patients and their family environment. The individualized intervention derived from the ETNA3 study used in this research paper allowed the tasks to be constantly updated by the trainers, who always focused on patients’ needs. This work encourages health professionals to consider their patients’ needs and preferences for building person-centered care based on best practices. However, this interventional rehabilitation program requires the presence of expert specialists to direct and personalize the treatment sessions. The small number of specialists assigned to meet the steadily increasing demand on the part of patients has forced them to opt for group interventions, reducing treatment effectiveness.
Our study has certain limitations: 1) The study was conducted at a single center, meaning that the characteristics of the population may not be representative of the entire Mexican population with AD. 2) This program requires trained personnel to conduct the sessions and needs committed caregivers to accompany and stimulate the patient during the various stages of the disease. 3) The post-treatment evaluations of the outcomes of the program did not observe statistically significant differences, due to the sample size and the fact that the program had to be interrupted due to the health emergency caused by Covid-19.
The strengths of our study include follow-up for an extended period (twelve months) focusing on each patient’s specific needs and those of their caregivers. This study was an evidence-based intervention.
CONCLUSION
This study describes the feasibility and acceptability of a personalized intervention program based on the ETNA 3 study in Mexican subjects with mild dementia and their caregivers. Although the sample size did not yield positive results concerning the effectiveness of the intervention, several subjects reported improved cognition and function. Additional trials that consider the need for trained therapists and caregiver support to achieve tangible cognitive and functional changes are warranted.